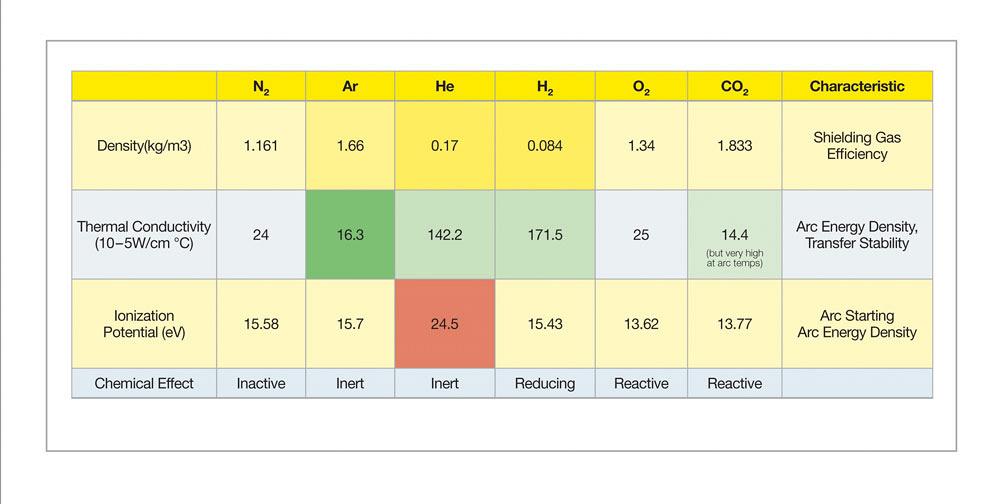
Welding stainless steel requires selecting shielding gases that preserve its metallurgical composition and associated physical and mechanical properties. Common shielding gas elements for stainless steel include argon, helium, oxygen, carbon dioxide, nitrogen, and hydrogen, (see Figure 1). These gases are combined in varying proportions to accommodate the needs of different transfer modes, wire types, base alloys, desired bead profiles, and travel speeds.
Gases for Short-Circuit Transfer GMAW
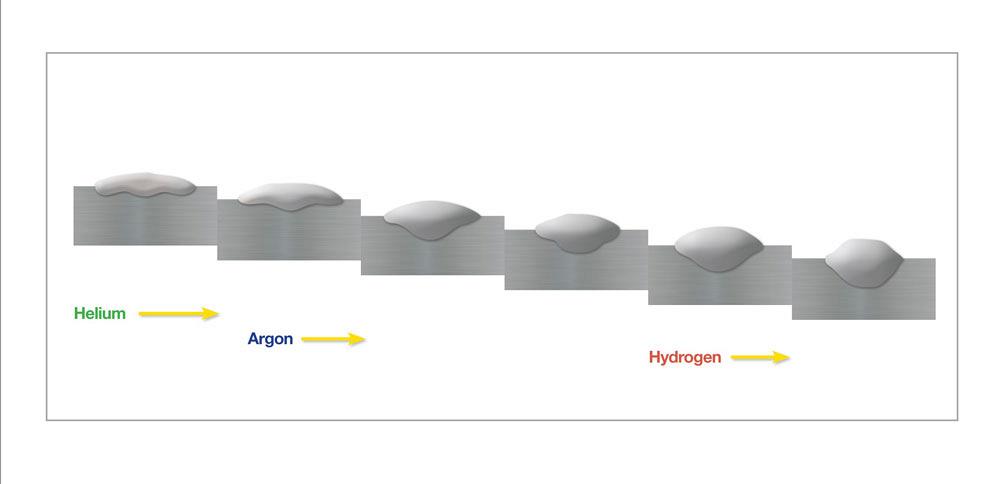
Because of stainless steel’s poor thermal conductivity and the relatively “cool” nature of short circuit transfer gas metal arc welding (GMAW), this process requires a “trimix” gas that contains a blend of 85 to 90 per cent helium (He), up to 10 per cent argon (Ar) and 2 to 5 per cent carbon dioxide (CO2). A common tri-mix blend contains 90 per cent He, 7-1/2 per cent Ar, and 2-1/2 per cent CO2. Helium’s high ionization potential promotes arc starting after the short circuit; coupled with its high thermal conductivity, using He will increase the fluidity of the weld pool. The Ar component of a trimix provides general shielding of the weld puddle, while the CO2 acts as a reactive component to stabilize the arc (see how different shielding gases affect the bead profile in Figure 2).
Some trimixes may use oxygen as a stabilizer, while others use a He/CO2/N2 blend to achieve the same results. Some gas distributors have proprietary gas blends that can deliver the benefits promised. Distributors also recommend these blends for other transfer modes, with equal results.
The biggest mistake fabricators make is attempting to short-circuit GMAW stainless steel with the same gas blend that they use for mild steel (75 Ar/25 CO2), typically because they don’t want an extra cylinder to manage. This blend contains too much carbon. In fact, any shielding gas for use with solid wires should contain a maximum of 5 per cent CO2. Using greater amounts yields a metallurgy that can no longer be considered an L grade alloy (L grade has less than 0.03 per cent carbon). With excess carbon in the shielding gas, chrome carbides could form, which would reduce corrosion resistance and mechanical properties. The surface of the weld will also appear sooty.
As a side note, when selecting metals for short-circuit GMAW on 300 series base alloys (308, 309, 316, 347), fabricators should choose an LSi grade. LSi filler has a low carbon content (0.02 per cent), which makes it particularly recommended when there is a risk of intergranular corrosion. The higher silicon content improves welding properties such as wetting to help flatten the crown of the weld and promote fusion at the weld toes.
Fabricators should be cautious when using the short-circuit transfer process. Because the arc extinguishes, incomplete fusion may result, making the process subpar for critical applications. In high-production situations, pulsed spray transfer would be a better option if the material can support its heat input (≥ 1/16 in. is about the thinnest possible material to weld with the pulsed spray mode). Where material thickness and welding position support it, spray transfer GMAW is preferred because it provides more consistent fusion.
Gases for Spray Transfer Modes
These higher-heat transfer modes do not require shielding gases with He. For spray transfer welding of the 300 series alloys, common choices are 98 per cent Ar and 2 per cent of active elements such as CO2 or O2. Some gas blends may also include a small about of N2. With its higher ionization potential and thermal conductivity, N2 promotes wetting and can enable faster travel speeds or improve penetration; it may also reduce distortion.
For pulsed spray transfer GMAW, 100 per cent Ar can be an acceptable choice. Because pulsing the current stabilizes the arc, the gas does not always require an active element.

Ferritic stainless steels and duplex stainless steels (with a 50/50 balance of ferrite and austenite) have a more sluggish puddle. For these alloys, a gas blend such as ~70 per cent Ar/~30 per cent He/2 per cent CO2 will promote better wetting and improve travel speeds (see Figure 3). It is possible to use a similar blend for welding nickel alloys, but it will cause the formation of nickel oxide on the surface of the weld (for example, the addition of 2 per cent CO2 or O2 would be enough to increase the oxide content, so fabricators should avoid them or be prepared to spend a lot of time grinding, as these oxides are so tough that a wire brush usually won’t remove them).
Gases for Cored Stainless Steel Wires
Fabricators use flux-cored stainless wires for out-of-position welding because the slag system in these wires provides a “shelf” that supports the weld puddle as it solidifies. Because the fluxing components can mitigate the effect of CO2, flux-cored stainless wires are designed for use with 75 per cent Ar/25 per cent CO2 and/or 100 per cent CO2 gas blends. While flux-cored wires may cost more per pound, it’s worth noting that higher all-position welding speeds and deposition rates may lower overall weld costs. Also, flux-cored wires use a conventional constant voltage DC output, and basic welding systems cost less and are less complex than pulsed GMAW systems.
Gases for GTAW
For 300 and 400 series alloys, 100 per cent Ar remains the standard choice for gas tungsten arc welding (GTAW). During GTAW of some of the nickel alloys, especially with a mechanized process, a small amount of hydrogen (up to 5 per cent) may be added to improve travel speeds (note that nickel alloys, unlike carbon steels, are not susceptible to hydrogen cracking).
For welding super duplex and hyper duplex stainless steels, 98 per cent Ar/2 per cent N2 and 98 per cent Ar/3 per cent N2 are good choices, respectively. Helium can also be added to improve wetting at about the 30 per cent level. When welding super duplex or hyper duplex stainless steel, the objective is to produce a joint with a balanced microstructure of approximately 50 per cent ferrite and 50 per cent austenite. Because microstructure formation depends on the cooling rate, and because the TIG weld puddle cools quickly, too much ferrite would remain when using 100 per cent Ar. When a gas blend with an N2 component is used, the N2 becomes stirred into the weld pool and promotes austenite formation.

Backing Gases
Stainless steel requires protecting both sides of the joint to produce finished welds with maximum corrosion resistance. Failure to protect the back side results in “sugaring,” or an extreme amount of oxidation that can cause a weld failure.
Tightly butted joints with consistently excellent fit-up or situations where the back of the joint is tightly contained may not require backing gas. Here, the primary issue is preventing the heat-affected zone from becoming excessively discolored because of oxide buildup, which would then need mechanical removal. Technically, if backside temperatures exceed 500 degrees F, a shielding gas is required. However, a more conservative approach is 300 degrees F as the threshold. Ideally, the backing should be below 30 PPM O2. The exception would be if the back side of the weld would be gouged, ground, and welded for a full-penetration weld.
The two backing gases of choice are N2 (least expensive) and Ar (more expensive). For small components or when a source of Ar is readily available, using that gas may be more convenient and not worth the cost savings of N2. Up to 5 per cent hydrogen can be added to reduce oxidization. A variety of commercial options are available, but homemade backing devices and purge dams are common.

The addition of 10.5 percent or more chromium is what gives stainless steel its stainless properties. Preserving those properties requires selecting the correct shielding gas for welding and good techniques to protect the back side of the joint. Stainless steel is expensive, and it’s being used for a good reason. There’s no point in trying to cut corners when it comes to shielding gases, or when selecting filler metals for that matter. As such, it always makes sense to work with a knowledgeable gas distributor and filler metal expert when selecting gases and filler metals for welding stainless steel.
Martin Denault is an application specialist, CWI, for ESAB Specialty Alloys.



